
Introduction
All the constituents of the endocannabinoid system (ECS) are widely expressed throughout the adult nervous system, but they are also highly present during gestation in the developing central nervous system. A particularly interesting observation is that the cannabinoid receptor type 1 (CB1 receptor) is expressed in two very different neuronal subpopulations: in inhibitory GABAergic neurons and in excitatory glutamatergic neurons. This implies that CB1 receptors have divergent roles in physiological processes throughout the entire organism. In addition, pathological dysregulations of the ECS may occur in either or both of these neuronal populations. Consequently, it is very critical to determine dysregulations at the exact neuronal level in order to be able to draw valid conclusions about the pathological processes. Such conditions include pathological states such as obesity, epilepsy, depression and anxiety disorders.
Key Points
- The ECS is activated on demand and thereby executes distinct functions in different behaviours.
- Dysregulations of the ECS may occur differentially, in a neuronal subtype specific manner. Thus, to draw valid conclusions, it is crucial to make an exact determination of the dysregulated neuronal subpopulation. The ECS can be over or underexpressed.
State of the Art
It has been known for several years that CB1 receptors are expressed in numerous brain regions but also in different neuronal subpopulations, including GABAergic and glutamatergic neurons [1]. Recently, it has been recognized that CB1 receptors are also present in serotonergic, noradrenergic and cholinergic neurons [2-4], but even dopaminergic neurons may contain CB1 receptors [4]. At the synaptic level, endocannabinoids are involved in retrograde signalling processes, where endocannabinoids are released from the postsynapse and, after crossing the synaptic cleft, they bind to pre-synaptic CB1 receptors, leading to the suppression of neurotransmitter release. As CB1 receptors are present on the synaptic terminals of different neurotransmitters, the physiological and pathophysiological consequences of retrograde endocannabinoid-mediated suppression of neurotransmission may be rather divergent, depending on the exact neurotransmitter that is modulated.
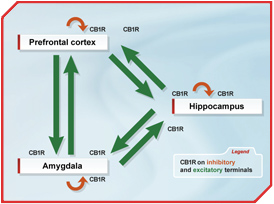 [Click to enlarge]
[Click to enlarge]
This particular feature of the ECS is illustrated in the endocannabinoid-mediated control of seizure threshold. In a rat model of febrile seizures, CB1 receptors are up-regulated specifically in hippocampal GABAergic interneurons upon prolonged hyperthermia in early postnatal stages, leading to a life-long decrease in GABA transmission. This dysregulation lowers the seizure threshold, making these animals prone to epilepsy [5]. A follow-up study showed that pharmacological blockade of CB1 receptors during the time of hyperthermia inhibited the emergence of febrile seizures in adulthood [6]. On the other hand, it was shown that lack of CB1 receptors specifically in cortical glutamatergic neurons also leads to an increased susceptibility to seizure-inducing agents, such as kainic acid, while the loss on GABAergic neurons had no effect on seizure susceptibility [7]. Postmortem analysis of human tissues from subjects with temporal epilepsy showed a specific decrease in hippocampal CB1 receptors in glutamatergic neurons, while no change in hippocampal GABAergic interneurons was observed [8]. Thus, these are intriguing results, indicating that depending on the site of dysregulation of the ECS, either blocking or enhancing endocannabinoid signalling may be of therapeutic benefit [9].
A dichotomy of endocannabinoid function is emerging for other processes. 1-During neural development, CB1 receptors are present on cortical glutamatergic neurons. Here, the functions include processes such as proliferation of neural progenitor cells, neuronal migration and axon fasciculation [10]. In GABAergic interneurons, CB1 receptors do not serve an apparent function in proliferation and migration, but rather in the growth cone and during synaptogenesis [11]. 2-In endocannabinoid-controlled fear extinction, CB1 receptors on cortical glutamatergic neurons play a crucial role [12]. However, data on CB1 receptor function on GABAergic interneurons is still pending for further evaluation, but preliminary results suggest that CB1 receptor function on this neuronal population might have the opposite effect. Further studies in other behavioural paradigms, such as anxiety and stress coping, will help validate whether CB1 receptor function is dichotomized in GABAergic and glutamatergic neurons.
But it will certainly be a challenging task to evaluate the specific function of CB1 receptors in a particular neuronal subpopulation within a distinct brain region. An illustration of this is endocannabinoid-controlled fear extinction, where the complete loss and the glutamatergic-specific loss of CB1 receptor leads to impaired fear extinction. However, considering the complex expression of CB1 receptors in neuronal networks involved in fear extinction (Figure), sophisticated genetic experiments will be required to pinpoint in detail CB1 receptor function to a particular behaviour.
Priorities for Future Studies
In any investigation addressing possible dysregulations of the ECS in the central nervous system, it will be very important to detail the sites of dysregulation. It will be rather straightforward to localize CB1 receptor expression and quantify over or underexpression in a particular neuronal subpopulation, but it will be very difficult to draw strong conclusions on dysregulated endocannabinoid levels, as endocannabinoids diffuse within 50-80 micrometers and influence a broad area where CB1 receptors are present. Further investigations on dysregulated ECS may focus on anxiety, stress coping, obesity and depression-like behaviours in animal models.
Due to the fact that CB1 receptors are present at a myriad of different nerve terminals in the brain, the question arises as to how this system is able to regulate specific behaviours, as it apparently does. One hypothesis is that the ECS is active only in neuronal networks that fire and are activated. Thus, this neuromodulatory system is active on demand at particular circuits and thereby executes specific functions. It will be important to decipher such endocannabinoid-regulated neural circuits.
References
- Marsicano G and Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 1999; 11: 4213-25.
- Degroot A, Kofalvi A, Wade MR, et al. CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol Pharmacol 2006; 70: 1236-45.
- Haring M, Marsicano G, Lutz B, et al. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 2007; 146: 1212-9.
- Oropeza VC, Mackie K and Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res 2007; 1127: 36-44.
- Chen K, Ratzliff A, Hilgenberg L, et al. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron 2003; 39: 599-611.
- Chen K, Neu A, Howard AL, et al. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci 2007; 27: 46-58.
- Monory K, Massa F, Egertova M, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006; 51: 455-66.
- Ludanyi A, Eross L, Czirjak S, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci 2008; 28: 2976-90.
- Lutz B and Monory K. Soothing the seizures of children. Nat Med 2008; 14: 721-2.
- Mulder J, Aguado T, Keimpema E, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A 2008; 105: 8760-5.
- Berghuis P, Rajnicek AM, Morozov YM, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 2007; 316: 1212-6.
- Kamprath K, Plendl W, Marsicano G, et al. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of CRH signaling. Genes brain Behav 2008; in press.



